Absolute Zero Is What Temperature On The Fahrenheit Scale
Espiral
Apr 07, 2025 · 5 min read
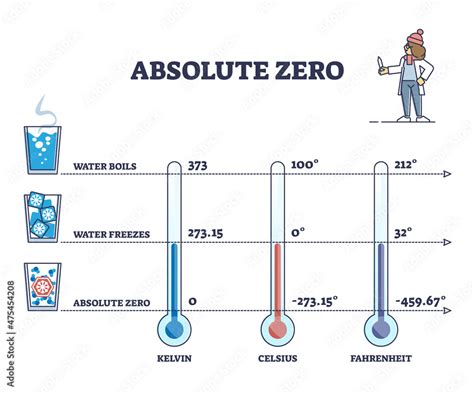
Table of Contents
Absolute Zero: What Temperature is it on the Fahrenheit Scale?
Absolute zero, the point at which all molecular motion ceases, is a concept of fundamental importance in physics and thermodynamics. It represents the lowest possible temperature, a theoretical limit that can never truly be reached, although scientists constantly strive to get closer. But what exactly is absolute zero on the Fahrenheit scale, and what are the implications of this fascinating point? Let's delve into the details.
Understanding Absolute Zero
Before we tackle the Fahrenheit conversion, it's crucial to grasp the concept of absolute zero itself. It's not merely the absence of heat; it's the point where all matter possesses its minimum possible energy. At this temperature, atoms and molecules would essentially stop moving, possessing zero kinetic energy. This state is hypothetical, as the laws of quantum mechanics prevent complete stillness. Even at absolute zero, there's a residual energy known as zero-point energy.
This concept is integral to understanding various scientific phenomena, including the behavior of gases, the efficiency of engines, and the properties of materials at extremely low temperatures. The quest to reach absolute zero has driven significant advancements in cryogenics and related technologies.
The Kelvin Scale: The Absolute Temperature Scale
The most straightforward way to express absolute zero is using the Kelvin scale. This absolute temperature scale, unlike Celsius or Fahrenheit, sets its zero point at absolute zero. Therefore, absolute zero is 0 Kelvin (0 K). This makes the Kelvin scale extremely convenient for scientific calculations and thermodynamic applications.
The Kelvin scale's simplicity is precisely why it is the preferred scale for scientific work involving temperature. There are no negative Kelvin temperatures—it starts at the theoretical limit and progresses upwards.
Converting to Fahrenheit: The Calculation
Now, let's address the main question: what is absolute zero on the Fahrenheit scale? To determine this, we need to use the conversion formula between Kelvin and Fahrenheit. The formulas are as follows:
- Kelvin to Celsius: °C = K - 273.15
- Celsius to Fahrenheit: °F = (°C × 9/5) + 32
Let's perform the calculation step-by-step:
-
Kelvin to Celsius: We start with absolute zero in Kelvin, which is 0 K. Using the formula, we get: °C = 0 K - 273.15 = -273.15 °C
-
Celsius to Fahrenheit: Now, we convert the Celsius value to Fahrenheit: °F = (-273.15 °C × 9/5) + 32 = -459.67 °F
Therefore, absolute zero is -459.67 °F.
The Significance of -459.67 °F
This seemingly simple numerical value holds immense scientific significance. It represents the theoretical lower limit of temperature within the framework of classical thermodynamics. Reaching absolute zero would imply a complete cessation of all thermal motion, an event that remains beyond our current technological capabilities.
The pursuit of temperatures approaching absolute zero has led to breakthroughs in various fields. Researchers utilize cryogenic techniques to study materials at extremely low temperatures, observing unusual quantum effects and developing new technologies. These advancements have implications for:
-
Superconductivity: Certain materials exhibit superconductivity at temperatures near absolute zero, allowing for the flow of electric current with virtually no resistance. This has significant potential for energy transmission and storage.
-
Quantum Computing: Quantum computers rely on maintaining extremely low temperatures to prevent the loss of quantum coherence, a critical requirement for their operation.
-
Medical Imaging: Cryogenic techniques are employed in medical imaging modalities like MRI (Magnetic Resonance Imaging), where superconducting magnets require ultra-low temperatures for operation.
-
Space Exploration: Understanding the behavior of materials at extremely low temperatures is essential for designing spacecraft and equipment capable of withstanding the harsh conditions of space.
Approaching, But Never Reaching, Absolute Zero: The Third Law of Thermodynamics
The Third Law of Thermodynamics states that it is impossible to reach absolute zero in a finite number of steps. This is not just a technological limitation; it's a fundamental principle of physics. As a system approaches absolute zero, the entropy (a measure of disorder) approaches a constant minimum value. The closer you get, the more energy—and therefore, effort—is required to reach the next increment lower. It's an asymptotic approach, meaning you can get infinitely close, but never actually reach the limit.
This law doesn't diminish the importance of absolute zero as a theoretical concept. It simply emphasizes the fundamental limitations inherent in trying to manipulate thermal energy at its most extreme point.
Practical Applications of Near-Absolute Zero Temperatures
While achieving absolute zero is impossible, getting extremely close to it has profound technological implications. Here are some examples:
-
Cryopreservation: Freezing biological samples at extremely low temperatures allows for long-term storage, preserving cells and tissues for future use in medical research and transplantation.
-
High-Precision Measurements: Near-absolute zero temperatures are crucial for extremely precise measurements in various scientific experiments. The reduced thermal noise allows for greater accuracy and sensitivity.
-
Advanced Materials Research: Investigating the properties of materials near absolute zero allows researchers to discover and develop new materials with extraordinary properties, like those exhibiting high-temperature superconductivity.
-
Laser Technology: Cryogenically cooled lasers produce highly stable and coherent beams, necessary for various applications, from telecommunications to scientific research.
Beyond Absolute Zero: Negative Temperatures?
While the term "negative temperature" might seem contradictory to the concept of absolute zero, it's a well-defined term in statistical mechanics. It doesn't mean a temperature lower than absolute zero; instead, it refers to a specific type of inverted population in a system, where more particles occupy higher energy states than lower ones. This is a very specific scenario achievable only in carefully controlled quantum systems and doesn't contradict the Third Law of Thermodynamics.
Conclusion: Absolute Zero – A Cornerstone of Physics and Technology
Absolute zero, represented as 0 K or -459.67 °F, is not just a numerical value; it's a pivotal concept in physics and thermodynamics. While the unattainability of absolute zero is guaranteed by the Third Law of Thermodynamics, the relentless pursuit of extremely low temperatures has fueled technological progress across numerous fields. From superconductivity to quantum computing and cryopreservation, our ability to approach absolute zero continues to shape scientific discovery and technological innovation. The quest to understand and harness the properties of matter at these incredibly low temperatures remains a vibrant area of research, promising further advancements in years to come. The understanding of absolute zero is fundamental to our grasp of the universe's most fundamental laws and our continued ability to push the boundaries of scientific knowledge.
Latest Posts
Latest Posts
-
East India Company Definition Ap World History
Apr 09, 2025
-
To Enlist Those Supporting The Cause
Apr 09, 2025
-
Mexican Border City Of Ciudad Juarez
Apr 09, 2025
-
The Shame Of The Cities By Lincoln Steffens
Apr 09, 2025
-
Battle Of New Orleans Apush Definition
Apr 09, 2025
Related Post
Thank you for visiting our website which covers about Absolute Zero Is What Temperature On The Fahrenheit Scale . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.