Hcl Is A Compound Or Element
Espiral
Mar 31, 2025 · 5 min read
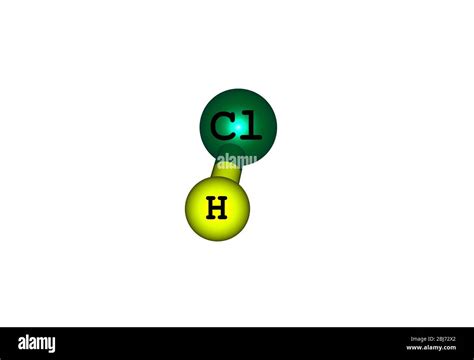
Table of Contents
Is HCL a Compound or an Element? Understanding Chemical Compounds
Understanding the fundamental building blocks of matter is crucial in chemistry. This article delves into the classification of substances as elements or compounds, focusing specifically on hydrochloric acid (HCl). We'll explore the defining characteristics of both elements and compounds, examine the molecular structure of HCl, and definitively answer whether HCl is an element or a compound. We will also explore related concepts and address common misconceptions.
Elements: The Fundamental Building Blocks
Elements are pure substances that cannot be broken down into simpler substances by chemical means. They are the fundamental building blocks of all matter. Each element is defined by the number of protons in its atomic nucleus, which is called its atomic number. The periodic table organizes all known elements based on their atomic number and recurring chemical properties. Examples include hydrogen (H), oxygen (O), carbon (C), and iron (Fe). These elements exist as individual atoms or, in some cases, molecules composed of identical atoms (e.g., O2, diatomic oxygen).
Key Characteristics of Elements:
- Pure Substances: Elements consist of only one type of atom.
- Indivisible by Chemical Means: They cannot be broken down further through chemical reactions.
- Defined by Atomic Number: Each element has a unique atomic number representing the number of protons in its nucleus.
- Found on the Periodic Table: All known elements are systematically organized in the periodic table.
Compounds: Combining Elements
Compounds are pure substances formed by the chemical combination of two or more different elements in a fixed ratio. This combination involves the atoms of the constituent elements forming chemical bonds, which are strong forces of attraction holding the atoms together. These bonds can be ionic (involving the transfer of electrons) or covalent (involving the sharing of electrons). The properties of a compound are distinctly different from the properties of its constituent elements. For example, water (H₂O) is a liquid at room temperature, while hydrogen and oxygen are both gases.
Key Characteristics of Compounds:
- Chemical Combination: Compounds are formed by the chemical bonding of two or more different elements.
- Fixed Ratio: The elements in a compound are always present in a fixed and definite proportion by mass.
- Distinct Properties: The properties of a compound are different from the properties of its constituent elements.
- Decomposable by Chemical Means: Compounds can be broken down into their constituent elements through chemical reactions.
Hydrochloric Acid (HCl): A Detailed Examination
Hydrochloric acid, also known as muriatic acid, is a highly corrosive, strong mineral acid. Its chemical formula is HCl, indicating that it is composed of hydrogen (H) and chlorine (Cl) atoms. Crucially, these are different elements. The hydrogen and chlorine atoms are bonded together through a covalent bond, where they share electrons to achieve a stable electron configuration.
The Molecular Structure of HCl:
The HCl molecule is a diatomic molecule, meaning it is composed of two atoms: one hydrogen atom and one chlorine atom. The hydrogen atom shares its single electron with the chlorine atom, which has seven valence electrons. This sharing creates a single covalent bond, resulting in a stable molecule. The bond is polar because chlorine is more electronegative than hydrogen, leading to a slight negative charge on the chlorine atom and a slight positive charge on the hydrogen atom. This polarity significantly influences HCl's chemical behavior and its strong acidity.
Why HCl is a Compound, Not an Element:
Given the above, the answer is clear: HCl is a compound, not an element. It meets all the criteria for a compound:
- It is composed of two different elements: Hydrogen and chlorine.
- These elements are chemically bonded: They are linked by a covalent bond.
- It has properties distinct from hydrogen and chlorine: HCl is a strong acid, whereas hydrogen is a flammable gas, and chlorine is a toxic, greenish-yellow gas.
- It can be decomposed: HCl can be broken down into hydrogen and chlorine gases through electrolysis or other chemical processes.
Differentiating Compounds and Mixtures
It is important to distinguish between compounds and mixtures. While compounds are formed through chemical reactions and have a fixed composition, mixtures are physical combinations of substances that retain their individual properties. The components of a mixture can be separated by physical methods, like filtration or distillation, whereas compounds require chemical methods for separation. For example, saltwater is a mixture of sodium chloride (NaCl – a compound) and water (H₂O – a compound). The salt can be separated from the water through evaporation.
Common Misconceptions about HCl
There are some common misconceptions regarding HCl that need clarification:
- HCl as a Solution: Often, when discussing HCl, we refer to hydrochloric acid, which is an aqueous solution of HCl gas dissolved in water. The HCl itself is still a compound; the solution is a mixture of HCl and water.
- Confusion with Hydrogen Chloride Gas: Hydrogen chloride (HCl) exists as a gas at room temperature and standard pressure. While it’s the same chemical compound, the state of matter doesn't change its classification as a compound.
- Thinking it's a single element due to the formula's appearance: The formula HCl might appear simple, but it denotes a chemical bond between two distinct elements. It's not a single element, but a combination of them.
Conclusion: HCl's Definitive Classification
In conclusion, hydrochloric acid (HCl) is unequivocally a compound, not an element. It is formed by the chemical combination of two different elements, hydrogen and chlorine, bonded together in a fixed ratio through a covalent bond. Its properties are distinctly different from those of its constituent elements, further solidifying its classification as a compound. Understanding this fundamental distinction is critical for comprehending chemical reactions, molecular structure, and the behavior of matter. The simplicity of its formula shouldn't overshadow the fundamental chemical processes that make it a compound.
Latest Posts
Latest Posts
-
Why Do You Put A Christmas Tree Up
Apr 02, 2025
-
Aaron Rodgers Ever Won A Superbowl
Apr 02, 2025
-
How Many Acres Is Cincinnati Zoo
Apr 02, 2025
-
Where Is Grand Bahama Island Located
Apr 02, 2025
-
Antipas Died Where He Was Exiled
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about Hcl Is A Compound Or Element . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
