What Determines The Properties Of A Protein
Espiral
Mar 31, 2025 · 6 min read
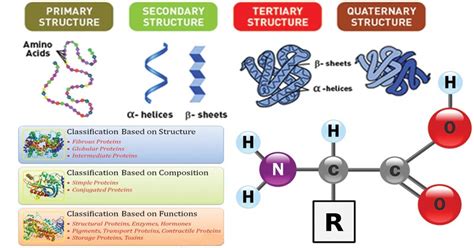
Table of Contents
What Determines the Properties of a Protein?
Proteins are the workhorses of the cell, carrying out a vast array of functions crucial for life. Their diverse roles, from catalyzing biochemical reactions to providing structural support, stem from their unique properties. But what exactly determines these properties? The answer lies in a complex interplay of factors, starting at the level of the amino acid sequence and extending to the protein's three-dimensional structure and its interactions with its environment.
The Primary Structure: The Foundation of Protein Properties
The fundamental determinant of a protein's properties is its primary structure: the linear sequence of amino acids. This sequence, dictated by the genetic code, is a precise blueprint that dictates all subsequent levels of protein organization. Each amino acid possesses unique chemical characteristics, including its:
-
Side chain (R group): This is the variable part of the amino acid, and its properties (hydrophobic, hydrophilic, charged, etc.) are key to shaping the protein's overall behavior. Hydrophobic side chains tend to cluster together in the protein's interior, away from water, while hydrophilic side chains are often found on the surface, interacting with the aqueous environment. Charged side chains can form ionic bonds, contributing to the protein's stability and interaction with other molecules.
-
Size and shape: The size and shape of the side chain also influence how the protein folds and interacts with other molecules. Bulky side chains can sterically hinder folding, while smaller side chains allow for greater flexibility.
-
Chemical reactivity: Some amino acid side chains are more chemically reactive than others. These reactive side chains can participate in covalent modifications, affecting the protein's function and lifespan. For example, cysteine residues can form disulfide bonds, stabilizing the protein's three-dimensional structure.
The Impact of Amino Acid Sequence on Protein Function
The specific arrangement of these amino acids within the sequence directly influences the protein's function. Even a single amino acid substitution can have drastic consequences, as seen in sickle cell anemia, where a single amino acid change in hemoglobin alters its shape and function, leading to severe health problems. The sequence determines:
-
Enzyme activity: The precise arrangement of amino acids in the active site of an enzyme determines its substrate specificity and catalytic efficiency.
-
Binding affinity: Proteins interact with other molecules, such as ligands, DNA, or other proteins. The amino acid sequence dictates the binding site's shape and chemical properties, determining the protein's affinity for its target.
-
Protein stability: The amino acid sequence influences the protein's overall stability, determining its resistance to denaturation (unfolding) and degradation. Proteins with high stability are more likely to function correctly for extended periods.
-
Post-translational modifications: The sequence can dictate where and how post-translational modifications (PTMs), such as phosphorylation, glycosylation, or ubiquitination, occur. PTMs can significantly alter the protein's properties, including its activity, localization, and lifespan.
Secondary Structure: Local Folding Patterns
Once synthesized, the polypeptide chain begins to fold into local structures known as secondary structures. These structures are stabilized by hydrogen bonds between the backbone atoms of the amino acid residues. Common secondary structures include:
-
α-helices: A right-handed coiled structure stabilized by hydrogen bonds between the carbonyl oxygen of one amino acid and the amide hydrogen of an amino acid four residues away. The properties of the side chains influence the stability and propensity of a region of the polypeptide chain to form an α-helix. Certain amino acids are helix-forming (e.g., alanine, leucine), while others are helix-breaking (e.g., proline, glycine).
-
β-sheets: Extended structures formed by hydrogen bonding between adjacent polypeptide chains (parallel or antiparallel). The side chains extend above and below the sheet, influencing its overall properties. β-sheets are often found in proteins involved in structural roles.
-
Loops and turns: These less-ordered regions connect α-helices and β-sheets, often playing a crucial role in protein function by forming binding sites or participating in catalytic activity. Their flexibility is essential for protein dynamics and function.
The arrangement and proportions of these secondary structures significantly influence the protein's overall shape and properties. For example, proteins rich in α-helices tend to be more flexible, while those rich in β-sheets tend to be more rigid.
Tertiary Structure: The Three-Dimensional Arrangement
The tertiary structure refers to the overall three-dimensional arrangement of the polypeptide chain, including the spatial positioning of all its secondary structural elements. This structure is determined by a complex interplay of various forces, including:
-
Hydrophobic interactions: Hydrophobic amino acid side chains cluster together in the protein's interior, minimizing their contact with water. This hydrophobic effect is a major driving force in protein folding.
-
Hydrogen bonds: Hydrogen bonds form between various polar side chains and the polypeptide backbone, contributing to the stability of the tertiary structure.
-
Ionic bonds (salt bridges): Electrostatic interactions between oppositely charged side chains can stabilize the protein's structure.
-
Disulfide bonds: Covalent bonds between cysteine residues can form strong cross-links, further stabilizing the protein's tertiary structure.
-
Van der Waals forces: Weak, short-range attractive forces between atoms contribute to the stability of the overall structure.
Domains and Motifs
Proteins often contain distinct structural and functional units called domains. Domains are independently folding units that can have specific functions, such as binding to a particular ligand or catalyzing a specific reaction. The arrangement and interactions of domains within the protein determine its overall function. Furthermore, recurring patterns of secondary structure elements, called motifs, often play critical roles in protein function.
Quaternary Structure: Multi-subunit Complexes
Some proteins consist of multiple polypeptide chains, also known as subunits, interacting to form a larger complex. This arrangement is called the quaternary structure. The interactions between subunits are similar to those stabilizing tertiary structure: hydrophobic interactions, hydrogen bonds, ionic bonds, and disulfide bonds. The quaternary structure significantly influences the protein's overall properties, including its stability, function, and regulation. Examples include hemoglobin (tetramer) and many enzymes with multiple subunits.
Post-Translational Modifications and Environmental Factors
The properties of a protein aren't set in stone after it folds. Post-translational modifications (PTMs), such as phosphorylation, glycosylation, ubiquitination, and acetylation, can alter a protein's properties dramatically. These modifications can affect the protein's activity, localization, stability, and interactions with other molecules. Furthermore, the cellular environment, including pH, temperature, ionic strength, and the presence of other molecules, also affects protein structure and function. Changes in these conditions can lead to protein denaturation or conformational changes, altering its activity.
Predicting Protein Properties from Sequence
While our understanding of protein structure and function has advanced significantly, predicting a protein's properties solely from its amino acid sequence remains a challenging task. The complexity of the folding process and the subtle influence of various interactions make accurate prediction difficult. However, computational methods, such as homology modeling and ab initio prediction, are continually improving, allowing us to gain more insights into the relationship between sequence and structure-function relationships.
Conclusion: A Complex Interplay
The properties of a protein are not determined by a single factor but by a complex interplay of its amino acid sequence, its various levels of structure, post-translational modifications, and its environment. Understanding these factors is critical for advancing our knowledge of biology and for developing new therapeutics targeting protein function. Research continues to unravel the intricate details of protein folding and function, leading to exciting advancements in various fields, from drug discovery to bioengineering. The remarkable diversity of protein functions stems from this precise interplay of multiple influences, ensuring the intricate orchestration of life's processes.
Latest Posts
Latest Posts
-
Who Is The Unionist Party In Government
Apr 02, 2025
-
Why Do You Put A Christmas Tree Up
Apr 02, 2025
-
Aaron Rodgers Ever Won A Superbowl
Apr 02, 2025
-
How Many Acres Is Cincinnati Zoo
Apr 02, 2025
-
Where Is Grand Bahama Island Located
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about What Determines The Properties Of A Protein . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
